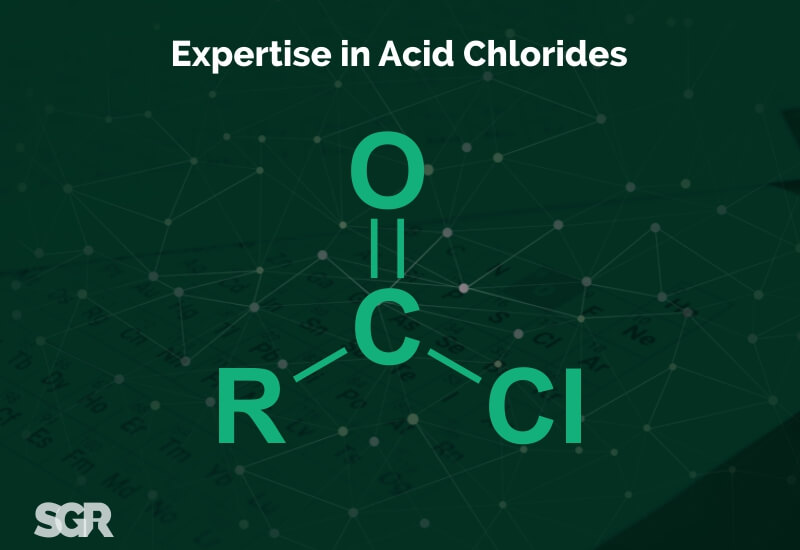
Expertise of SGRL in Acid Chlorides Manufacturing and Chlorination Capabilities
February 01, 2019
SGRL has broad expertise in handling of the following reactions and processes. Using our vast experience with different chemistries, we can undertake custom/contract manufacturing for you.
- Chlorination
- Grignard Reactions
- Hydrogenation / Reduction
- Oxidation
- Bromination
- Esterification
Chlorination for Acid Chlorides
Carboxylic acids react with Thionyl Chloride (SOCl2) to form Acid chlorides. During the reaction, the hydroxyl group of the carboxylic acid is converted to a chlorosulfite intermediate making it a better leaving group. The chloride anion produced during the reaction acts a nucleophile.
Mechanism of Chlorination to Manufacture Acid Chlorides
There is a distinct mechanism to make acid chlorides using thionyl chloride compounds. The reaction mechanism behind an acid chloride reaction, is called nucleophilic acyl substitution.
Let's break down the steps in sequence progressing in this reaction:
Step 1: We call the oxygen atom, in the alcohol group, a nucleophile. A nucleophile is a molecule or ion that will donate its electron pair to form a chemical bond. This nucleophile attacks the carbon atom, an electrophile, in the carbonyl group (CO). An electrophile is a molecule that accepts an electron pair to form a chemical bond. When the nucleophile attacks the electrophile, the result is an intermediate formed with our friend thionyl chloride.
Step 2: Following this attack, the intermediate collapses. This causes the removal of the chlorine atom as a leaving group from thionyl chloride. A leaving group is any fragment (molecule or atom) that leaves with its electrons. Once the leaving group is removed, a chlorosulfite ion is formed.
Step 3: A second nucleophilic attack on the carbonyl group of the chlorosulfite ion occurs. The chlorine atom, a leaving group, bonds to the carbon atom. Note that this carbon atom is an electrophile. Once bonded, an intermediate is formed.
Step 4: The leaving group is removed and intermediate collapses. This leads to the production of by-products such as hydrochloric acid and SO2 gas. This gases are then scrubbed into their relevant or suitable media to form by-products such as HCL Acid or sodium bi-sulphite solution.
Leading Products of SGRL in Acid Chlorides:
2-Furoyl chloride CAS No: 527-69-5
4-Chlorobutyryl chloride CAS No: 4635-59-0
O-Acetylsalicyloyl chloride CAS No: 5538-51-2
Cyclopropanecarbonyl chloride CAS No: 4023-34-1
SGRL is leading in manufacturing and exporting of Advanced Pharmaceutical Intermediates
Shree Ganesh Remedies Limited, with its chemical reaction technologies, is now leading manufacturer and exporter of Drug Intermediates and Advanced Pharmaceutical Intermediates. SGRL has list of leading companies as clients to represent in the CPHI 2018 in December at Delhi. Exhibiting in CPHI delhi is the first powerful step for us on such large platform for pharmaceutical chemicals and ingredients.
Chemical reaction technologies like Chlorination with thionyl chloride and chlorine, Bromination, Hydrogenation / Reduction, Oxidation, Grignard Reactions, Acylation allows SGRL to manufacture products with customization and contract chemistry can be developed.
SGRL is leading in many Pharmaceutical Intermediates in the World with its expertise in production. Some other key products are:
Bis(2-chloroethyl) amine hydrochloride CAS 821-48-7
Cyclopropane carbonyl chloride CAS 4023-34-1
2-Methoxyphenothiazine CAS 1771-18-2